
No prescriptions for Merck’s Renflexis (infliximab biosimilar) were noted, despite the product having been launched in July 2017, about halfway through the study period. During this time frame, 857 patients from 73 of the RISE rheumatology practices (30% of the total number of practices) were found to have initiated prescriptions for Pfizer’s infliximab biosimilar, Inflectra.

The study looked at data from September 1 st 2016 (before the first biosimilar prescription) through March 3 1st, 2018. Katherine Liao, the RISE registry was used to analyse the uptake of infliximab biosimilars across rheumatology indications, following their initial approval and launch. Schmajuk noted that while targeted studies are needed, factors potentially driving unique practice-specific patterns of drug prescription include practice size (smaller practices tend to be more variable), patient insurance access, or even possibly, a “social networking” effect in which trainees adopt their mentor’s prescription preferences. When asked to speculate on this drastic variability in prescription patterns across rheumatology practices, Dr. The variability between practices was staggering, with the overall biologic/tofacitinib prescription rate ranging from 19-79% and the proportions of individual non-TNF inhibitor drugs ranging from 1% to over 35% of the total prescribed drugs. Key opinion leaders (KOLs) interviewed by GlobalData expressed their interest and excitement about this class of drugs, but these data from the RISE registry suggest this perspective extends to community practices as well.Ī particularly intriguing figure presented during the session compared the biologic/tofacitinib prescription patterns across all 87 individual practices. The fact that tofacitinib use has eclipsed that of the IL-6 inhibitor class highlights just how enthusiastic US physicians are about JAK inhibitors. Interestingly, the percentage of patients prescribed tofacitinib was nearly equal to the combined percentages of patients prescribed tocilizumab (2.7%) and rituximab (1.9%).

The next most commonly prescribed drugs were abatacept (4.7%) and tofacitinib (4.2%). As expected, TNF inhibitors were the most commonly prescribed drug class, representing 27% of all prescriptions.
During the year-long study period, 40% of patients were prescribed a biologic or tofacitinib. Prescription data between June 2016 and July 2017 were analysed in 79,027 patients from 87 rheumatology practices. Gabriela Schmajuk, used data from the RISE registry to capture national trends in the prescription of non-TNF inhibitor, second-line drugs. Both provided interesting insight into the treatment of RA in the US.
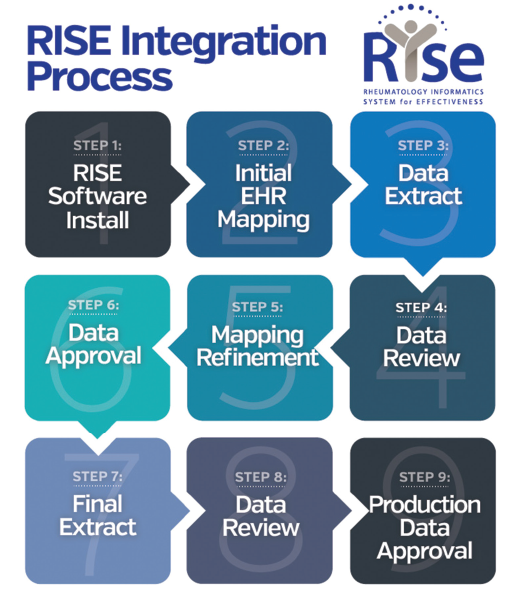
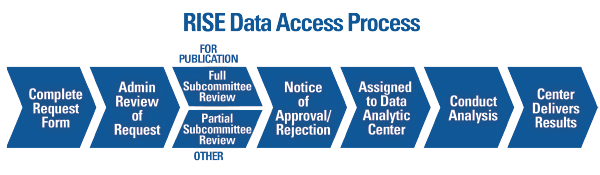
Two of these analyses were presented at the 2018 ACR/ARHP Annual Meeting in an oral abstract session dedicated to the implementation of big data in health services research. Data from the RISE registry are also made available to researchers who are using the burgeoning database to analyse the changing treatment landscape of rheumatoid arthritis (RA) and other rheumatic diseases in the US. These data can then be used by physicians to evaluate their quality of care as well as to fulfil the reporting requirements of the Centers for Medicare and Medicaid Services. Created in 2016 by the American College of Rheumatology (ACR), the Rheumatology Informatics System Effectiveness (RISE) registry is a HIPAA-compliant, qualified clinical data registry that passively collects electronic health record (EHR) data from participating rheumatology practices.


 0 kommentar(er)
0 kommentar(er)
